Anti-tumor activity of cabozantinib by FAK down-regulation in human oral squamous cell carcinoma
DOI:
https://doi.org/10.3329/bjp.v11i1.24001Keywords:
Cabozantinib, FAK down-regulation, Oral squamous cell carcinoma, TumorAbstract
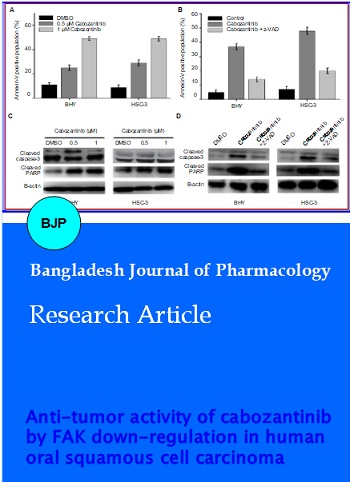
Cabozantinib is a tyrosine kinase inhibitor involved in inhibition of cell proliferation and colony formation. We studied anti-cancer properties of cabozantinib in oral squamous cell carcinoma cells. The viability of BHY and HSC-3 cells decreased with increase in cabozantinib concentration and time. The proliferation of cell lines was affected by increasing concentration of cabozantinib from 0.3 to 1.2 ?M after 48 hours of treatment. The expression of MET and phosphorylated MET was not affected by cabozantinib treatment. Cabozantinib-treated cells when compared to control, showed concentration-dependent increase in BHY and HSC-3 cells during G2/M phase and decrease in S phase with increase in cabozantinib concentration. Annexin-V/propidium iodide double staining showed that cells with annexin-V increased with the increase in cabozantinib concentration. The expression of apoptosis related proteins cleaved caspase-3 and cleaved-PARP were increased with increase in cabozantinib concentration. It was also found that suppression of FAK activation and expression was dose dependent. The results from this study revealed that cabozantinib can be useful in developing a drug for effective treatment of oral squamous cell carcinoma cells.
Downloads
380
216 Read
174
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).
